Slawomir Filipek

Speaker of Workshop 4
Will talk about: Ligand binding and action of microswitches in G protein coupled receptors
Dr. Slawomir Filipek graduated at University of Warsaw, Poland in 1985 in the field of quantum chemistry. Then he started working with larger, macrocyclic molecules and obtained a Ph.D. degree at the same University in 1993 in theoretical chemistry,. Apart from modeling of organic molecules and investigating of their properties he also interested in much larger structures, biomolecules, and drug design techniques. For this part of research he collaborated with Pharmaceutical Institute in Warsaw. In 2001 he moved to Seattle, WA, to Kris Palczewski laboratory and participated in discovery of oligomeric structure of rhodopsin. He made an atomistic model of the rhodopsin oligomer which was later confirmed by experiments. Dr. Filipek was also working there on structure-function modeling of other proteins responsible for the vision processes as well as on other GPCRs.
After coming back to Poland in 2002 he started to head their own laboratory at International Institute of Molecular and Cell Biology in Warsaw. He also obtained habilitation degree in 2004. His group is working on (1) modeling of structures of GPCRs and ligand docking; (2) modeling of activation and deactivation processes of GPCRs; (3) investigation of influence of internal (mutations) and external (ion metals, pH etc) factors on stability of proteins; (4) modeling of gamma-secretase membrane complex and analysis of mutations responsible for onset of Alzheimer Desease.
Dr. Filipek is an author of over 70 papers in internationally recognized journals. They gained nearly 2000 citations since 2000 year what indicates that the scientific problems they focus on are still in the center of the current science.
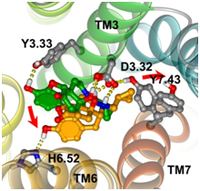 G protein coupled receptors (GPCRs) interact with very diverse sets of ligands which bind to the transmembrane segments and sometimes also to the receptor extracellular domains. Each receptor subfamily undergoes a series of conformational rearrangements leading to the binding of a G protein during the activation process. All GPCRs preserved the 7-TM scaffold during evolution but adapted it to different sets of ligands by structure customization. Binding of structurally different agonists requires the disruption of distinct intramolecular interactions, leading to different receptor conformations and differential effects on downstream signaling proteins. The dynamic character of GPCRs is likely to be essential for their physiological functions, and a better understanding of this molecular plasticity could be important for drug discovery. Experiments suggest that agonist binding and receptor activation occur through a series of conformational intermediates. Transition between these intermediate states involves the disruption of intramolecular interactions that stabilize the basal state of a receptor. Such profound changes are evoked by the action of molecular switches (microswitches). The switches proposed so far for different GPCRs include the “rotamer toggle switch” involving the CWxPxF sequence on helix TM6, the switch based on the NPxxY(x)(5,6)F sequence linking helices TM7 and H8, the “3 7 lock” interaction connecting TM3 and TM7 (involving Schiff base-counterion interaction in rhodopsin), and the “ionic lock” linking transmembrane helices TM3 and TM6 and employing the (E/D)RY motif.
G protein coupled receptors (GPCRs) interact with very diverse sets of ligands which bind to the transmembrane segments and sometimes also to the receptor extracellular domains. Each receptor subfamily undergoes a series of conformational rearrangements leading to the binding of a G protein during the activation process. All GPCRs preserved the 7-TM scaffold during evolution but adapted it to different sets of ligands by structure customization. Binding of structurally different agonists requires the disruption of distinct intramolecular interactions, leading to different receptor conformations and differential effects on downstream signaling proteins. The dynamic character of GPCRs is likely to be essential for their physiological functions, and a better understanding of this molecular plasticity could be important for drug discovery. Experiments suggest that agonist binding and receptor activation occur through a series of conformational intermediates. Transition between these intermediate states involves the disruption of intramolecular interactions that stabilize the basal state of a receptor. Such profound changes are evoked by the action of molecular switches (microswitches). The switches proposed so far for different GPCRs include the “rotamer toggle switch” involving the CWxPxF sequence on helix TM6, the switch based on the NPxxY(x)(5,6)F sequence linking helices TM7 and H8, the “3 7 lock” interaction connecting TM3 and TM7 (involving Schiff base-counterion interaction in rhodopsin), and the “ionic lock” linking transmembrane helices TM3 and TM6 and employing the (E/D)RY motif.
To investigate the early activation steps concurrent to ligand binding we used opioid receptors. They belonging to the family A (rhodopsin-like) of GPCRs. For the important role they play in the human body in controlling pain and stress, modulating immune responses and developing addiction the opioid receptors were subject of numerous investigations. We chose a set of rigid ligands with the structural motif of tyramine because ligand flexibility would obscure the very first structural movements induced upon ligand binding. On the basis of conducted molecular dynamics simulations we propose that agonists and antagonists bind to Y3.33 but only agonists are able to move deeper into the receptor binding site and to reach H6.52. The movement from Y3.33 to H6.52 induces breaking of the TM3-TM7 connection D3.32-Y7.43 (“3-7 lock” switch). We also observed a concerted motion of W6.48 and H6.52 suggesting existence of an extended “rotamer toggle switch”. Simultaneous action of both switches, the “3-7 lock” and the “rotamer toggle switch”, implies a temporal but also spatial (an agonist linking H6.52 and D3.32) dependence between them and possibly other switches on a longer time scales.
References
1. M. Kolinski, S. Filipek, “Molecular Dynamics of mu Opioid Receptor Complexes with Agonists and Antagonists”, TOSBJ (2008) 2, 8-20.
2. M. Kolinski, S. Filipek, “Studies of the Activation Steps Concurrent to Ligand Binding in OR and OR Opioid Receptors Based on Molecular Dynamics Simulations”, TOSBJ (2009) 3, 51-63.
3. M. Kolinski, S. Filipek, “Structurally similar pair of agonist and antagonist of kappa opioid receptor studied by molecular dynamics simulations”, J. Mol. Model. (2010) in press.