Robert E. Marc

Speaker of Workshop 2
Will talk about: Mining retinal connectomes
Robert E. Marc (BSc Cum Laude, University of Texas / El Paso 1971; PhD Neuroscience, University of Texas / Houston) is Professor of Ophthalmology, the Calvin and JeNeal Hatch Presidential Endowed Chair and Director of Research for the John A. Moran Eye Center at the University of Utah. With Harry G. Sperling, he produced the first chromatic cone maps of the retina. With W.K. Stell at UCLA he began the process of mapping retinal pathways using cell-specific markers and transmission electron microscopy (TEM), providing the first neurochemical definition of feedback systems in the vertebrate retina. He joined the University of Texas / Houston as an assistant professor (1978), became the Robert Greer Professor of Biomedical Sciences in 1986, and remained there until 1993, when he joined the Moran Eye Center at the University of Utah. Since then, work in the Marc laboratory led to the development of computational molecular phenotyping, new functional probes of neuronal excitation, discovery of extreme neural and glial remodeling in retinal degenerations, and completion of the first retinal connectome. Current research include mining of connectome maps, development of comprehensive proteomic and metabolic profiling tools, and characterization and management of pathologic retinal remodeling. His research has been funded by the NIH since 1978. In 1996, he and Ann Torrence co-founded Signature Immunologics, Inc.
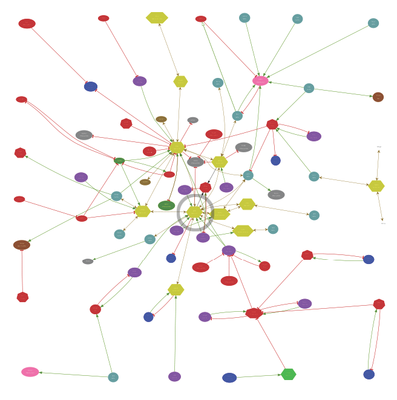 neural networks. It demarcates viewing and analysis from capture and hosting and permits applying image transforms in real-time. It also permits the fusion of registered thin-section optical molecular data with TEM image data, augmenting the collection of cell classification metadata. Connectome dataset RC1 was imaged at 2 nm resolution, balancing competing constraints of large-area sampling and fine-scale cell association maps (subclasses of chemical synapses, gap junctions, adherens junctions, organelle patterning). We use a crowd-sourcing strategy for annotation with Viking. This leads to rapid assembly of directed cyclic network graphs, dynamically visualized via a web-services Viz application that also provides network validation, error discovery and correction. The network graph below illustrates the associations of a single class A-II glycinergic amacrine cell (C467, circled) in the rabbit retina tracked through four synaptic “hops”. Even if automated tracking and annotation were viable, a Viz-like application would still be critical for finding and correcting network errors. Moreover, crowd-sourcing enables the discovery of novelty (connective, associative and ultrastructural), which automated tools have yet to achieve. In a year of analysis, mining connectome RC1 has uncovered new synaptic pathways and topologies, new non-neural activities, and new signaling states. Intensive mining of connectomics datasets provides the unique opportunity to build realistic system models based on complete synaptic partner maps. Support: NEI EY02576, NEI EY015128, P30EY014800, NIH T32DC008553, NSF 0941717, Research to Prevent Blindness. Disclosure: REM is a principal of Signature Immunologics, Inc.
neural networks. It demarcates viewing and analysis from capture and hosting and permits applying image transforms in real-time. It also permits the fusion of registered thin-section optical molecular data with TEM image data, augmenting the collection of cell classification metadata. Connectome dataset RC1 was imaged at 2 nm resolution, balancing competing constraints of large-area sampling and fine-scale cell association maps (subclasses of chemical synapses, gap junctions, adherens junctions, organelle patterning). We use a crowd-sourcing strategy for annotation with Viking. This leads to rapid assembly of directed cyclic network graphs, dynamically visualized via a web-services Viz application that also provides network validation, error discovery and correction. The network graph below illustrates the associations of a single class A-II glycinergic amacrine cell (C467, circled) in the rabbit retina tracked through four synaptic “hops”. Even if automated tracking and annotation were viable, a Viz-like application would still be critical for finding and correcting network errors. Moreover, crowd-sourcing enables the discovery of novelty (connective, associative and ultrastructural), which automated tools have yet to achieve. In a year of analysis, mining connectome RC1 has uncovered new synaptic pathways and topologies, new non-neural activities, and new signaling states. Intensive mining of connectomics datasets provides the unique opportunity to build realistic system models based on complete synaptic partner maps. Support: NEI EY02576, NEI EY015128, P30EY014800, NIH T32DC008553, NSF 0941717, Research to Prevent Blindness. Disclosure: REM is a principal of Signature Immunologics, Inc.